DOWNLOADS:
Why Hypochlorous Acid?
Hypochlorous Acid is one of the most natural and effective known biocides known to man. It eradicates all bacteria, mycobacteria, spores, fungi, viruses – even the tough Clostridium difficile – within 15 seconds. It disinfects 80 to 100 times better than bleach (Sodium Hypochlorite) and is 100% safe.
Hypochlorous acid oxidizes (explodes) the cell wall of all pathogens causing necrosis (rupturing of the cell) or apoptosis (programmed cell death) and destroys them. Though viruses are not technically living organisms, they too are destroyed by hypochlorous acid.
Despite this destructive potential, our bodies use this defense throughout life.
Hypochlorous acid is naturally produced by our white blood cells and is an essential part of our Immune System.
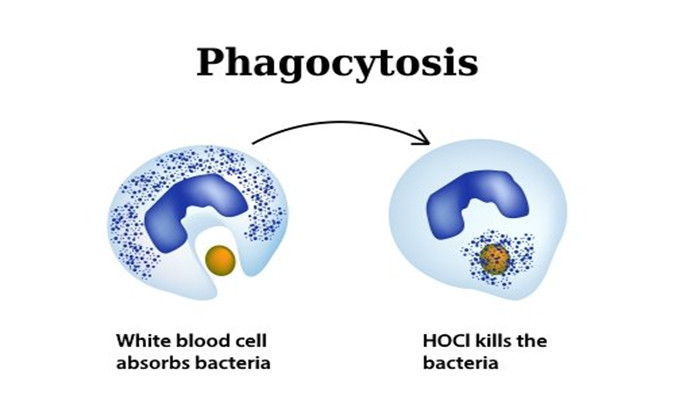
This process is called phagocytosis and is one of humans' most symbiotic actions – white blood cells release this natural oxidant to eliminate pathogens while being inherently harmless, unlike most other disinfectants which are toxic.
When a wound breaks human skin, it creates a gateway for harmful pathogens to invade human cells. Neutrophils, which are a type of white blood cell, travel in the blood to the site of the wound where the pathogens are invading. When an invading pathogen or infection threatens a human cell, the body's immune system responds by destroying the pathogen before it can harm the cell. The invading pathogens are engulfed by white blood cells through a process called phagocytosis. Once engulfed, the white blood cell produces an oxidant, hypochlorous acid. Hypochlorous Acid is a biocide and kills the microbial pathogen within milliseconds of contact. This antimicrobial process is called the Oxidative Burst Pathway.
You can use hypochlorous for surface disinfection! Hypochlorous can be sprayed liberally on all surface types and pathogens are neutralized within seconds.
Hypochlorous can also be used for dental waterlines – it allows you to eliminate biofilm and legionella with no side effects!
Other uses: impression disinfectant, instrument soak, ultrasonic baths, disinfect root canals, removing biofilms from implant surfaces, mouthrinse (particularly effective post-surgical), and endodontic irrigation.
Hypochlorous acid does not cause irritation to eyes and skin. Even it were ingested it causes no harm. Because it is so safe, it is the ideal sanitizer for direct food sanitation and food contact surfaces. It is also ideal in healthcare where it is used for wound cleansing, eye drops, and patient room disinfection replacing toxic chemicals such as bleach and quaternary ammonium (quats). Sanitation chemicals distributed in concentrated form are toxic and can be hazardous. Contact with skin or inhalation of fumes can cause irritation. These risks do not exist with hypochlorous acid.
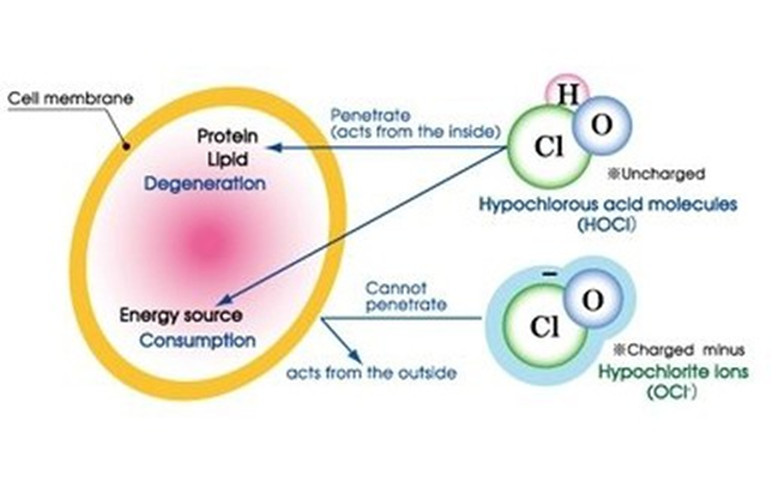
How does HOCL kill mocrobial pathogens?
The Molecule of hypochlorous acid is HOCl. This molecule is unique in that it is neutrally charged unlike hypochlorite (OCl-) which is negatively charged. So why is this important? Disinfectants and microbial pathogens interact with each other similar to magnets. If you bring together two negatively charged magnets, they will repel each other. Bacteria and hypochlorite (OCl- aka. bleach) are both negatively charged and behave like two negatively charged magnets repelling each other. Hypochlorous acid (HOCl) is neutrally charged and is not repelled by bacteria. HOCl easily penetrates the walls of the bacteria and destroys them with its strong oxidation potential.
Applications
- Sanitising Tunnels
- Large Area Misting
- Produce Processing
- Meat Processing
- Food Contact Surfaces
- Biofilm Control
- Sanitation
- Medical